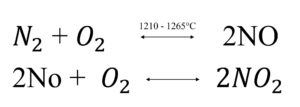Conflicts over water
The major river water conflicts in India are:
- Sharing of Cauvery water by Karnataka and Tamil Nadu
- Krishna water by Karnataka and Andhra Pradesh
- The Bhavani water by Tamil Nadu and Kerela
- Satluj- Yamuna water by Punjab and Haryana.
- The Tungabhadra Water Dispute between Andhra Pradesh and Karnataka
- The Aliyar and Bhivani water dispute between Tamil Nadu and Kerala
- The Godavari river water dispute between Andhra Pradesh, Madhya Pradesh, Chhattisgarh, Orissa and Karnataka.
- The Mahi river dispute between Gujrat, Rajasthan and Madhya Pradesh.
- The Ravi and Beas river water dispute between Punjab, Haryana,himachal Pradesh, Rajasthan, Jammu and Kashmir and Delhi.
So far following tribunals have been appointed to resolve interstate water conflicts:
- The Godavari Water Disputes Tribunal
- The Krishna Water Disputes Tribunal
- The Narmada Water Water Disputes Tribunal
- The Ravi and Beas Water Tribunal
- The Cauvery Water Tribunal
- Vansadhara Water disputes tribunal
- Mahadayi Water Disputes Tribunal
Pollution
Definitions
Pollution is the unfavourable alteration of our environment , largely because of human activites.
It is defined as an undesirable change in the physical, chemical or biological characteristic of our air, land and water causing harmful effect on our life or that of other desirable species and cultural assets.
Pollution means addition of any foreign material like inorganic, organic, biological or radiological or any physical change occurring in nature, which may harm or affect living organisms directly or indirectly, immediately or after a long time.
Pollution is the release of harmful substances or energy into the environment by man in quantities that damage health and resources.
Types of Pollutants
Classification According to Environment.
When different spheres of the environment are affected by pollution, they can be categorized as
(i) Air pollution
(ii) Water pollution
(iii) Soil or land pollution
Classification According to the pollutant. The pollution caused by pollutants may be of a number of types.
(i) Radioactive Pollution
(ii) Sewage Pollution
(iii) Pesticide pollution or Biocide pollution
(iv) Noise pollution
(v) Thermal Pollution
(vi) Plastic Pollution
(vii) Marine Pollution
(viii) Smoke Pollution
(ix) Smog pollution
(x) Chemical Pollution
(xi) Industrial Pollution
(xii) Metal Toxicity Pollution
(xiii) Drug Pollution
(xiv) Estuarine or Oceanic Pollution
(xv) Biological pollution
(xvi) Silt Pollution
(xvii) Soap and Detergent Pollution
(xviii) Effluent Pollution
(xix) Acid Rain pollution
(xx) Oil Pollution
Air Pollution
Depending upon the generation of different air pollutants, they are grouped as
1. Primary Pollutants
2. Secondary Pollutants
1. Primary Pollutants
A primary pollutant can be defined as a harmful chemical that directly enters the air as a result of either man made or natural activities.
There are 5 major primary air pollutants which together contribute more than 90% of world’s air pollution.i.
i. Carbon Monoxide (CO)
It is colourless, odourless, tasteless gas,chemically inert under normal conditions of temperature and pressure. It has no effect at normal concentration (0.1 ppm) but at higher concentrations it seriously affects the human metabolism.
Sources
- Natural processes such as volcanoes, natural gas emission.
- Electrical discharge during cloud forming, seed germination march gas production etc. contribute in atmosphere though in small amounts.
- Transportation contribute 64% of CO in air.
- Forest fires and agricultural burning contributes about 17% of CO in atmosphere.
- Industrial processes such as electric furnance and blast furnances in iron and steel industry, petroleum refining, paper industry, gas manufacture and coal mining etc. causes about 9.6% of CO in air.
Effects
- Reduces the oxygen carrying capacity of the blood by selectively combining with haemoglobin (Hb) forming carbozyhaemoglobin (COHb). This causes giddiness, laziness and exhaustion.
- It reduces vision and causes cardiovascular disorders.
- CO is a very dangerous asphyxiant and its high levels are fatal to human life.
Another oxide of carbon is carbon dioxide. It is the basic end product obtained on the burning fossil fuels, paper, leaves and other carbon containing material. Carbon dioxide is used by plants for photosynthesis. Although carbon dioxide has no direct effect on health, but with higher concentrations it causes global warming, acid rain and greenhouse effect.
ii. Nitrogen Oxides
The group include eight possible oxides nitrogen, NO, NO2, and N2O primarily involved in air pollution. NO is a colourless, odourless gas, but NO2 is reddish brown and have suffocating odour. NO and NO2 are formed as:
The formation of NO occurs at high temperature. The second reaction is also favoured at high temperature about 1100C°. NO2 is also formed by photolytic reaction.
Sources
Activities such as agriculture, fossil fuel combustion and industrial processes are the primary cause of the increased nitrous oxide concentrations in the atmosphere. Together these sources are responsible for 77% of all human nitrous oxide emissions. Other sources include biomass burning (10%), atmospheric deposition (9%) and human sewage (3%).
The largest human source of nitrous oxide emissions is from agriculture which accounts for 67%. Agriculture creates both direct and indirect emissions. Direct emissions come from fertilized agricultural soils and livestock manure (42%). While indirect emissions come from runoff and leaching of fertilizers (25%). Agriculture creates 4.5 million tonnes of nitrous oxide per year.
The use of synthetic fertilizer for agriculture is a major source of nitrous oxide emissions. Fertilizers help feed plants by adding nitrogen directly to soils. But soil bacteria also take advantage of this extra nitrogen and use it to produce the energy they need to live and grow. Microbial processes of nitrification and denitrification produce nitrous oxide which is then released into the atmosphere.
The storage and handling of livestock manure is another direct source of agricultural nitrous oxide emissions. When the manure of livestock is not used as a fertilizer or left in fields during grazing, it has to be kept for treatment and disposal in animal waste management systems. Many of these systems create conditions that are favorable for nitrous oxide producing bacteria.
Fossil fuel combustion and industrial processes are an important source of nitrous oxide emissions. Industrial processes also causes nitrous oxide emissions. The two main industrial sources are the production of nitric and adipic acid. Nitric acid is an important ingredient for synthetic fertilizers, while adipic acid is primarily used for making synthetic fibers. For both of these acids, oxidization of nitrogen compounds during the production process creates nitrous oxide.
A substantial amount of nitrous oxide is caused by biomass burning, which accounts for 10% of human-caused emissions.1 Biomass burning is the burning of living and dead vegetation. In these fires, some of the nitrogen in the biomass and surrounding air is oxidized creating nitrous oxide emissions.
As with animal waste, human waste is a significant source of nitrous oxide emissions. Sewage plants and septic tanks are used to store and treat wastewater. Many of these systems create conditions that are favorable for nitrous oxide producing bacteria. Human sewage produces 3% of human emissions.
Apart from being created by human activities, nitrous oxide is also released into the atmosphere by natural processes. The Earth’s soil, oceans and atmosphere are all natural sources of nitrous oxide emissions.
Chemical reactions in the atmosphere produce a significant amount of nitrous oxide emissions. The atmosphere acts as a source for nitrous oxide through the oxidation of ammonia which creates 5% of emissions. Ammonia is a natural occurring gas in the atmosphere. The oceans, manure from wild animals as well as aging and rotting plants form the most important natural sources of ammonia in the air.
Effects
- Like CO, nitric oxide can also combine with haemoglobin and reduces the oxygen carrying capacity of blood.
- NO is moderately toxic as its concentration in air is not high.
- NO2 is more toxic, it irritates alveoli of the lungs and its high concentration may cause acute bronchitis.
iii. Sulphur oxides
It includes Sulphur dioxide and Sulphur trioxide. SO2 is a colourless gas having pungent and suffocating odour.
Sources
Most of the Sulphur oxides pollution (67%) is due to volcanic activities and other natural sources. Remaining emissions(33%) are due to industrialization and automobile pollution. Sulfur dioxide is also present in motor vehicle emissions, as the result of fuel combustion.
Effects
- It irritates the nose, throat, and airways to cause coughing, wheezing, shortness of breath, or a tight feeling around the chest. The effects of sulfur dioxide are felt very quickly and most people would feel the worst symptoms in 10 or 15 minutes after breathing it in.
- When sulfur dioxide combines with water and air, it forms sulfuric acid, which is the main component of acid rain.
- Plants are sensitive to high concentrations of Sulphur dioxide and suffer chlorosis, metabolic inhibition, plasmolysis and even death.
iv. Hydrocarbons
The gaseous and volatile hydrocarbons are mainly responsible for air pollution. Common hydrocarbons include methane, ethylene, acetylene, terpenes etc.
Sources
- Trees emit large quantities of hydrocarbons in the atmosphere.
- Anaerobic decomposition of organic matter by bacteria produces hydrocarbons.
- Production, Treatment, storage and distribution of fossil fuels, combustion processes, applications of volatile organic solvents and solvent containing products, industrial and biological processes.
- Mining and processing of coal mainly lead to methane emission, but also ethane and propane are produced in minor amounts.
- Production, as well as storage and distribution of liquid fossil fuels, comprise a large variety of activities that result in hydrocarbon emissions to the atmosphere. Crude oil production platforms on land or offshore are point sources of hydrocarbons such as methane, ethane, propane, butanes, pentanes, hexanes, heptanes, octanes and cycloparaffins. Processing of petroleum products, resulting in fuels, feedstock and primary petrochemicals, includes separation, conversion and blending, and leads to emissions.
Effects
- Hydrocarbons at high concentrations are carcinogenic and therefore harmful for the lungs.
- Aromatic hydrocarbons like benzene and toluene are more dangerous, inhalation of their vapour causes irritation of the mucuous membrane. Increased concentration of hydrocarbons vapours increases mucus irritation leading to blockage of the respiratory tract, as a result of which a person coughs continuously.
- Hydrocarbons react with nitrogen oxides and produce photochemical smog that caused irritation to the eyes, nose and throat, as well as respiratory distress.
- Plants exposed to high levels of hydrocarbons display yellowing of the leaves.
v. Particulates
These are small, solid particles and liquid droplets present in the atmosphere in fairly large numbers and sometimes pose serious air pollution problem. In size particulates ranges from 0.02 µ in diameter of 500 µ with lifetime varying from a few seconds to several months.
Sources
- The particulates can be organic or inorganic in nature.
- The natural particulate matter present in aerosol is known as organic particulate matter. For example, particulates that originate from volcanoes, dust storms, forest and grassland fires and living vegetation.
- Inorganic particulate matter mainly comprised, metal oxides formed during the burning of fossil fuels, industrial processes, vehicular exhaust and acid rain. During combustion of pyrites, coal iron oxide is formed and from its ash, calcium oxide is released into the atmosphere.
Effects
- Particle pollution exposure to a variety of health issues, including irritation of the eyes, nose and throat, coughing, chest tightness and shortness of breath, reduced lung function, irregular heartbeat, asthma attacks, heart attacks, premature death in people with heart or lung disease.
- Particulate matter adversely affects the plant kingdom. The deposition of toxic substances makes the soil unsuitable for plant growth. These particles also get deposited on plant leaves and block the stomata of the plant, thereby decreasing the rate of respiration and photosynthesis causing the vegetation to perish.
- The suspended particulate mater accelerates the corrosion of metals, It is more common in urban and industrial areas than in villages.
- They also corrode buildings and sculptures.
Particulates educec visibility by adsorbing and effectively scattering solar radiation.
They influence the climate through formation of clouds, rain and snow and acts as nuclei on which water condenses. Hence it also affects the nature of precipitation.
2. Secondary Pollutants
Secondary pollutants are formed by primary pollutants their reaction with normal atmospheric compounds. Ozone is a secondary pollutant formed by photochemical reaction between primary pollutants and natural atmospheric gas. Ozone affects the respiratory and nervous system. It also damages rubber products and textiles. PAN, a secondary pollutant is formed when hydrocarbon radicals react with nitrogen dioxide. Peroxy Acetyl Nitrate causes photochemical smog and also causes irritation of the eyes, nose, throat and respiratory distress. Disposition of the toxic secondary pollutants, either in gaseous form or as particulates on soil make the soil unsuitable for plant growth. Acid rain for longer period decreases the pH of the soil and makes it acidic. Particulates on the other hand, deposit on the leaves of plants and block their stomata. As a result the rate of photosynthesis and transportation decreases and leads to decreased crop yield and retarded growth. Cattle are also found to be affected by air pollution. Ozone and PAN irritates the eyes, impair vision and create breathing problems leading to asthma in human beings.
Common Air pollutants, their sources and effects of human beings
| Pollutant | Source | Pathological effect on human beings |
| Aldehydes | Thermal decomposition of oils, fats and glycerols. | Irritates nasal and respiratory tracts. |
| Ammonia | Explosives, dye-making, fertilizer plants and lacquers. | Inflames upper respiratory passage. |
| Arsenic | Process involving metal or acid containing arsenic soldering | Damages red blood cells, kidneys and causes jaundice. |
| Carbon monoxide | Burning of coal, gasoline motor exhaust. | Reduces oxygen carrying capacity of blood. |
| Hydrogen cyanides | Blast furnance, fumigation, chemical plants. | Interferes with nerve cells, produces dry throat, indistinct vision, headache. |
| Hydrogen sulphide | Refineries, chemical industries and bituminous fuels. | Causes nausea, irritation of eyes and throat. |
| Nitrogen oxide | Soft coal, automobile exhaust. | Inhibits cilia action so that soot and dust penetrated far into the lungs. |
| Phosgene | Chemical and dye-making industry. | Induces coughing, irritation and fatal pulmonary oedema. |
| Sulphur dioxide | Combustion of coal and oil. | Causes chest constriction, headache, vomiting and ultimately, death due to respiratory ailments. |
| Suspended particles (ash, soot, smoke, etc. ) | Incinerator and almost every manufacturing process. | Causes emphysema, irritation in the eyes and possibly, cancer. |