Protoplast Technology
The term protoplast was introduced by Hanstein in 1880. It refers to the cellular content excluding cell wall or can also be called as naked plant cell. It is the living matter enclosed by a plant cell membrane. The first isolation of protoplasts was achieved by Klercker (1892) employing a mechanical method. The application of protoplast technology for the improvement of plants offers fascinating option to compliment conventional breeding programs. The ability of isolated protoplasts to undergo fusion and take up macromolecules and cell organelles offers many possibilities in genetic engineering and crop improvement.
The experiments involving protoplast consist of three stages :
I. Protoplast isolation
II. Protoplast fusion
III. Development of regenerated fertile plants from the fusion product
I. Protoplast Isolation
Protoplasts are naked plant cells without cell wall, but they have plasma membrane and all other cellular components. They represent the functional plant cells but for the lack of the barrier, cell wall. Protoplasts of different species can be fused to generate a hybrid and this process is referred to as somatic hybridization (or protoplast fusion).
Protoplasts are isolated by
- Mechanical Method
- Enzymatic Method
1. Mechanical Method
Klercker in 1892 pioneered the isolation of protoplast by mechanical methods. In this method a small piece of epidermis from a plant is selected. The cells are subjected to plasmolysis. This causes protoplasts to shrink away from the cell walls. The tissue is then dissected to release the protoplasts.
Disadvantages
- It is restricted to certain tissues which have large vacuolated cells.
- Yield of protoplasts is generally very low.
- The method is tedious and laborious.
- Viability of protoplasts is low because of the presence of substances released by damaged cells.
2. Enzymatic Method
Cocking in 1960 demonstrated the possibility of enzymatic isolation of protoplasts from higher plants. Mechanical isolation method of protoplast are no more practically used. Protoplasts are routinely isolated by treating tissues with the mixture of cell wall degrading enzymes in solution, which contain osmotic stabilizer. The method involves the following steps.
- Sterilization of leaves
- Peeling off lower epidermis
- Incubation in enzyme solution
- Isolation and cleaning of the protoplast
The release of protoplasts is very much dependent on the nature and composition of enzymes used to digest the cell wall. There are three primary components of the cell wall which have been identified as cellulose, hemicelluloses and pectin substances. Cellulose and hemicelluloses are the components of the primary and secondary structure of cell wall, while pectin is a component of middle lamella that joins the cells. Pectinase mainly degrades the middle lamella, while cellulose and hemicellulose are required to digest the cellulosic and hemicellulose components of the cell wall respectively.
Different enzymes preparations are available in the market by the idea is to combine one middle lamella dissolving and one cell wall digesting enzymes in proper composition to achieve maximum protoplasts release from one gm material.
Following enzymes are used:
- Macerozymes R-10
- Cellulase- Onuzuka R-10
- Hemicellulose
- Pectinase
- Drieselase
A combination of these enzymes in a concentration of 0.5 – 2 % is used. In many cases only macerozyme and cellulose are sufficient to obtain protoplasts in significant number.
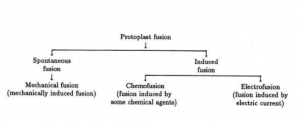
II. Protoplast Fusion
Protoplast fusion also called Somatic fusion is a type of genetic modification in plants by which two distinct species of plants are fused together to form a new hybrid plant with the characteristics of both.
There are two methods of Protoplast fusion:
- Spontaneous Fusion
- Induced Fusion
- Spontaneous Fusion
Protoplasts during isolation often fuse spontaneously and this phenomenon is called spontaneous fusion. Simply physical contact is sufficient to bring about the spontaneous fusion among the similar parental protoplasts. During the enzyme treatment for the isolation of protoplasts from adjoining cells fuse through their plasmodesmata to form a multinucleate protoplast. Electronic microscopic studies have shown that as the cell wall are enzymatically degraded, the plasmodesmatal connection between the adjacent cells enlarge due to removal of its constriction and the enlargement of pi fields. Eventually the greater enlargement of plasmodesmata allows the entry of organelles into neighboring cells. Spontaneous fusion is strictly intraspecific and gives rise to homokaryon.
- Induced Fusion
Fusion of freely isolated protoplast from different sources with the help of fusion inducing chemical agents is known as induced fusion. Some of the methods that have been employed are explained below:
i. Chemofusion
In this method protoplasts are fused by using chemical agents like NaNO, Calcium ions or Polyethylene glycol.
a) NaNO3 Treatment
Equal densities of protoplasts from two different sources are mixed and then centrifuged at 100g for 5 minutes to get a dense pellet. This is followed by addition of 4 ml of 5.5% sodium nitrate in 10% sucrose solution to resuspend the protoplast pellet. The suspended protoplasts are kept in water – bath at 35ºC for 5 minutes and again centrifuged at 200 g for 5 minutes. The pellet is once again kept in water bath at 30ºC for 30 minutes. The fusions of protoplast take place at the time of incubation. Finally, the protoplasts are plated in semisolid culture medium. The frequency of fusion is not very high in this method
b) Calcium ions at high ph
Keller and Melchers (1973) studied the effect of high ph and calcium ions on the fusion of tobacco protoplasts. In this method, isolated protoplasts are centrifuged for 3 min at 50xg in a fusion inducing solution of 0.5 M mannitol containing 0.05 M Cacl2.2H20 at a pH of 10.5.The centrifuge tubes containing the protoplasts are then incubated in a water bath at 37ºC for 40-50 min. After this treatment, 20-50 % of the protoplasts are involved in fusion.
c) Polyethylene glycol method
Kao and Michayluk and Wallin developed the PEG method of fusion of protoplasts. This one of the most successful techniques for fusing protoplasts. The protoplasts are suspended in a solution containing high molecular weight PEG, which enhances agglutination and fusion of protoplasts in several species. When sufficient quantities of protoplasts are available, 1ml of the protoplasts suspended in a culture medium are mixed with ml of 28-56% PEG (1500-6000 MW) solution. The tube is then shaken for 5 sec and allowed to settle for 10 min. The protoplasts are then washed several times by the addition of protoplast culture medium to remove PEG. The protoplast preparation is then resuspended in the culture medium. The PEG method has been widely accepted for protoplast fusion because it results in reproducible high frequency heterokaryon formation, low cytotoxicity tomost cell types and the formation of binucleate heterokaryons. PEG induced fusion is non specific and is therefore useful for interspecific,intergeneric or interkingdom fusions.